Project Charter
This project will provide a core framework to create interoperability between FAIR data sets across the pharmaceutical industry by identifying preferred vocabularies for community-wide standardization of core concepts.
The Challenge
The pharmaceutical industry faces significant challenges with information management and knowledge transfer throughout its entire range of operations.
Making information more accessible across a business is an overwhelming challenge facing companies, as the information is often fragmented in various silos. This challenge is made even more difficult by the large volumes of information generated, for example by automated R&D processes.
The challenges above are compounded when different pharmaceutical and other enterprises (e.g. CROs) and organizations (e.g. regulatory agencies) need to exchange data.
“FAIR silos”, namely resources complying with the FAIR principles but only meeting interoperability requirements within a given organization and organisational subunit, are emerging.
On the other hand, models and ontologies proliferate. Their diversity limits semantic interoperability, increases costs, creates semantic siloes and slows the path to data centricity in Life-Sciences organizations. Perhaps more critically, there is a lack of controlled definition around entities and the range of terms used to enumerate them. At a minimum, standardization is needed as far as vocabulary for core concepts is concerned.
Solution
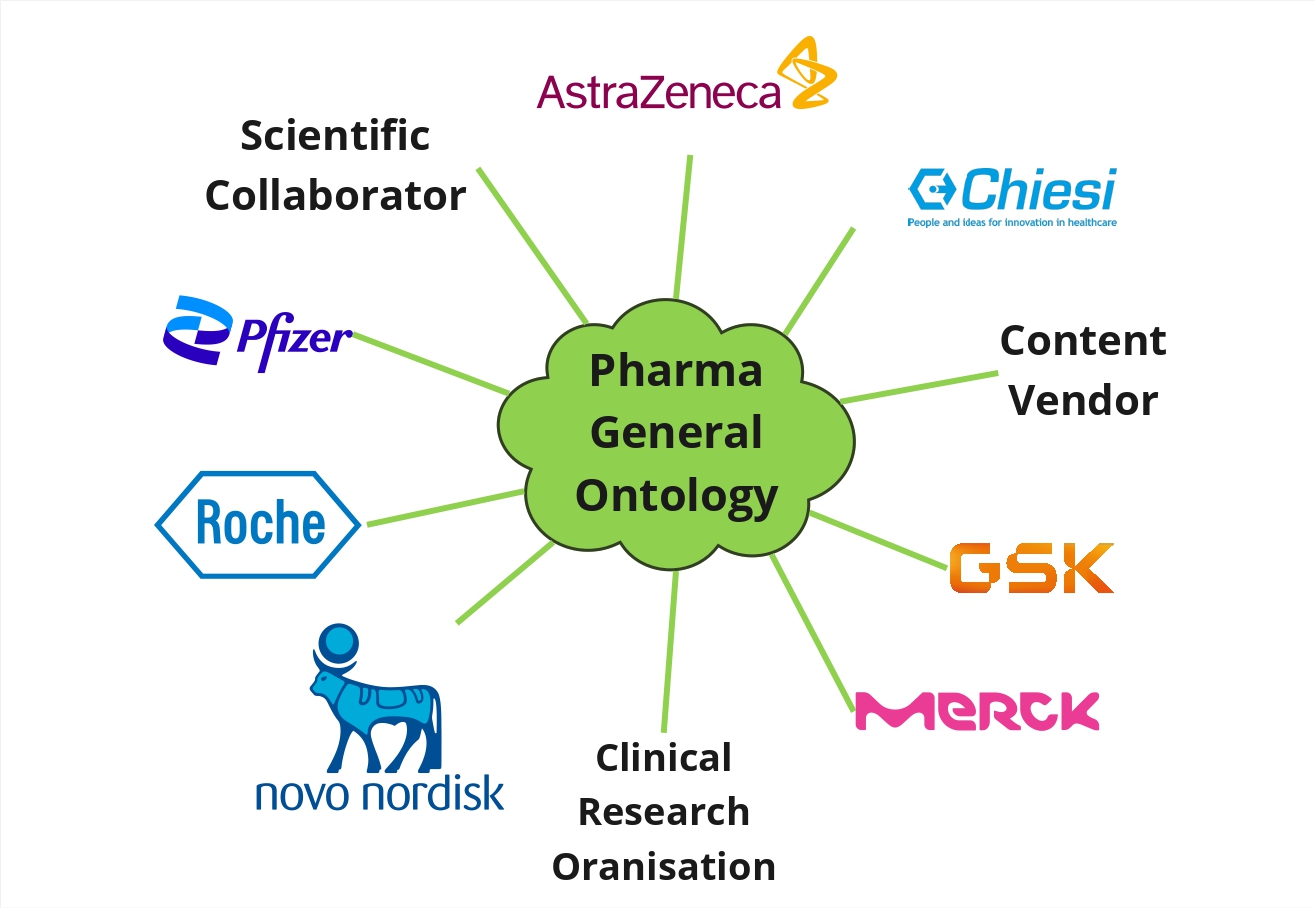
The purpose of PGO is to provide a lingua franca for the pharmaceutical sector. This will be achieved by experts designated by the Steering group which will, among others:
- Identify key concepts
- Select public resources that can provide identifiers for each concept.
PGO acts as “Rosetta Stone” enabling interoperability within and across organisations.
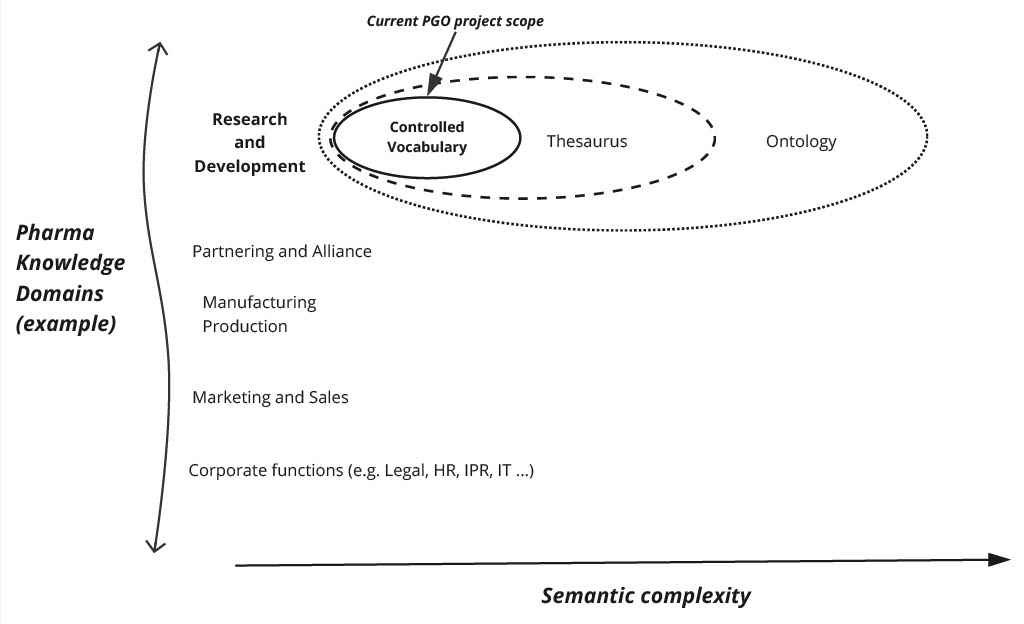
Future extension could include defining a minimal acceptable profile for each concept and the public vocabularies that can be used. While the whole of the medicinal product development life cycle is ultimately in scope, we will focus initially on R&D during the first phase of the project starting in April 2024. Creating a sustainability model to ensure the continuity of PGO as a reference data product through the Pistoia Alliance is a vital aspect of the project as well.
Get Involved
Talk to our project manager to learn more about phase 2 of this project and get involved
Contact Us