Project Charter
Our project aims to develop a pharmaceutical (CMC) process ontology, based on the ISA88/95 framework. This ontology will serve to standardize laboratory and plant production process recipes, and in turn, establish standardized definitions.
It will also facilitate digital technology transfers, and integrate with execution systems to capture structured process data for material lot genealogy tracking. This will lead to streamlined technology transfers, advanced process analytics, and ultimately, enhance efficiency and transparency throughout the pharmaceutical production lifecycle.</p>Chemical development has been supported by the ISA 88 standard for decades. A lot has changed in that time, and a more flexible, comprehensive representation of process data is needed. A contemporary ontology provides the data representation that is needed to facilitate data integration, data exchange and data insights.
The Pistoia Alliance has completed Phase 1 / Proof of Concept to build a pharmaceutical CMC process ontology based on the ISA88/95 framework.
The aim is to standardize laboratory and plant production process recipes to establish standardized definitions, facilitate digital technology transfers and integration with execution systems to capture structured process data for material lot genealogy tracking, streamline technology transfers, and advanced process analytics, and thereby enhance efficiency and transparency throughout the pharmaceutical production lifecycle.
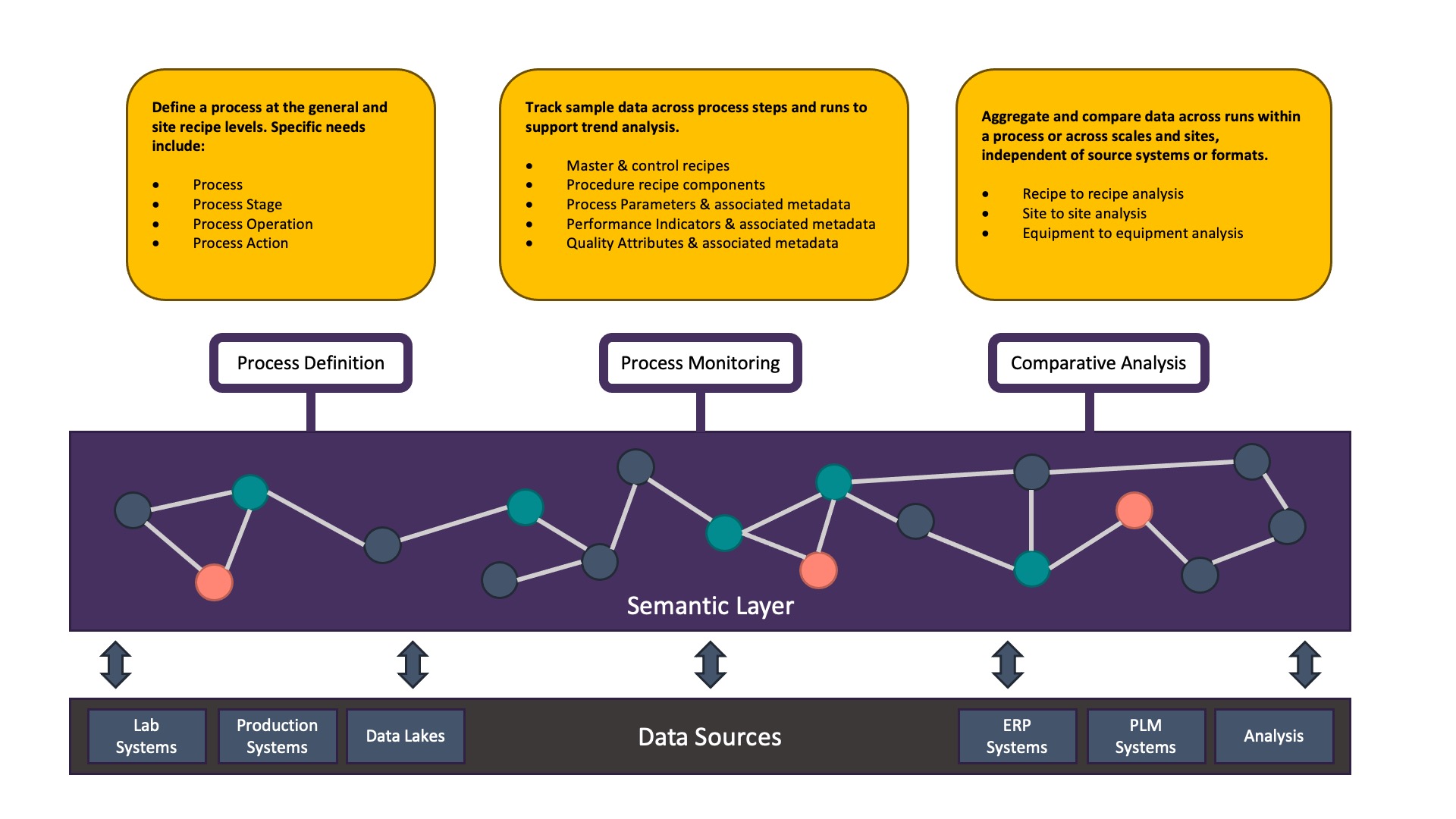
For this project’s initial phase, we worked on three use cases: Process Definition, Process Monitoring, and Comparative Analysis. The focus was on the processes for making the APIs and the drug products, initially focusing on biologics and synthetics.
Get involved in Phase 2
Talk to our project manager to learn more and get involved in Phase 2 of this project
Contact Us